PI: Zachary P. Smith, Department of Chemical Engineering, MIT
Abstract
Membrane-based gas separations are among the most energy-efficient separations in industry. These processes operate by selectively permeating certain components and rejecting others. Typically, smaller molecules permeate more quickly than larger molecules because of their faster diffusion rates. However, it is possible to engineer a “reverse-selective” membrane that permeates larger molecules and rejects smaller ones if the membrane material preferentially dissolves/adsorbs the larger components. To achieve this type of behavior, this proposal seeks to evaluate a polymer-nanoparticle hybrid membrane that preferentially dissolves/adsorbs heavier hydrocarbons (e.g., ethane, propane, butane) while rejecting methane. The gases of interest represent the major components found in associated natural gas in Russia. Today, associated gas is largely discarded and flared, so this proposal presents a membrane-based process and a materials design strategy to capture this valuable natural resource and eliminate on-site flaring that emits a significant amount of carbon into the atmosphere.
Report
- Project Title: Recovering hydrocarbons from glare gas with reverse-selective membranes
- Principal Investigator: Zachary P. Smith, Department of Chemical Engineering, Massachusetts Institute of Technology
- Grant Period: September 2017 – August 2018 (extended to 2/28/19)
This project’s aim was to develop “reverse selective” polymer-nanoparticle hybrid mixed- matrix membranes (MMM) that preferentially adsorb heavier hydrocarbons from associated gas. Specifically, we tested MMMs with metal-organic framework (MOF) nanoparticles dispersed in rubbery polymers that exhibit “reverse-selective” performance to assess the feasibility of our goal. An ideal MOF filler for making “reverse-selective” MMMs must preferentially adsorb heavier hydrocarbons while simultaneously having low diffusion selectivity for the same molecules. MOF MIL-101 contains coordinatively unsaturated open metal sites and large pore windows of approximately 12 Å and 16 Å [1]. Open metal sites provide exceptional adsorption selectivity for higher hydrocarbons over methane while the large pore windows allow for unhindered access of a variety of hydrocarbon gases into the framework [2]. Rubbery polymers (poly(dimethylsiloxane) (PDMS) and poly(ether-block-amide) (Pebax)) were used as the polymer matrix for the MMM because of their potential reverse-selective behavior [3].
1.Synthesis of MIL-101 (Cr) MOF nanoparticles
MIL-101(Cr) was synthesized using a modified hydrothermal protocol [1, 4]. Fig. 1 shows the TEM and SEM images of the synthesized MIL-101 (Cr) nanoparticles. They have multifaceted sphere morphology without significant agglomerations. The images also indicate that the MIL-101 (Cr) possesses a small particle size of about 60 nm with a narrow size distribution. The small and uniform particle size of the MIL-101 (Cr) nanoparticles are very important for the formation of good MMMs.
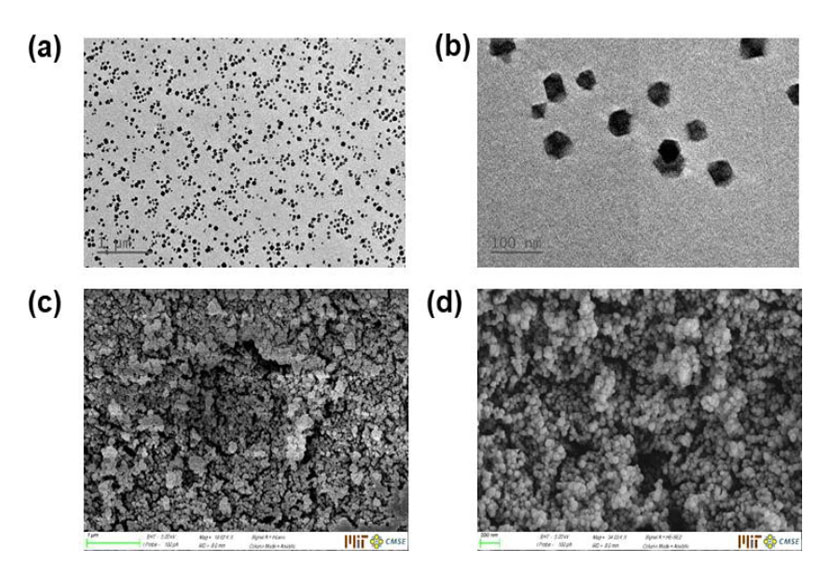
Fig. 1. TEM images (a, b) and SEM images (c, d) of the synthesized MIL-101 (Cr) nanoparticles.
The powder XRD pattern displayed in Fig. 2 (a) indicates that the synthesized MIL-101 (Cr) nanoparticles are well crystalline and all the peak positions are good agreement with the simulated and experimental XRD patterns of MIL-101 (Cr) [1]. The narrow peak with high intensity represents the crystalline nature of the MOF material. Fig. 2 (b) shows the N2 adsorption- desorption isotherm of MIL-101 (Cr) at 77 K. It has two secondary uptakes around p/p0 = 0.1 and p/p0 = 0.2, indicating the presence of two nanoporous windows in the framework. A high BET surface area of 3080.1 m2/g is achieved, which is higher than the reported values mainly because of its smaller particle size [1]. The FTIR spectra confirms the chemistry of the MIL-101 (Cr) framework (Fig. 2 (c)); while the TGA analysis clearly indicates that the MIL-101 (Cr) is thermally stable even up to 250 ° and it could be activated at relative high temperatures prior to membrane fabrication.
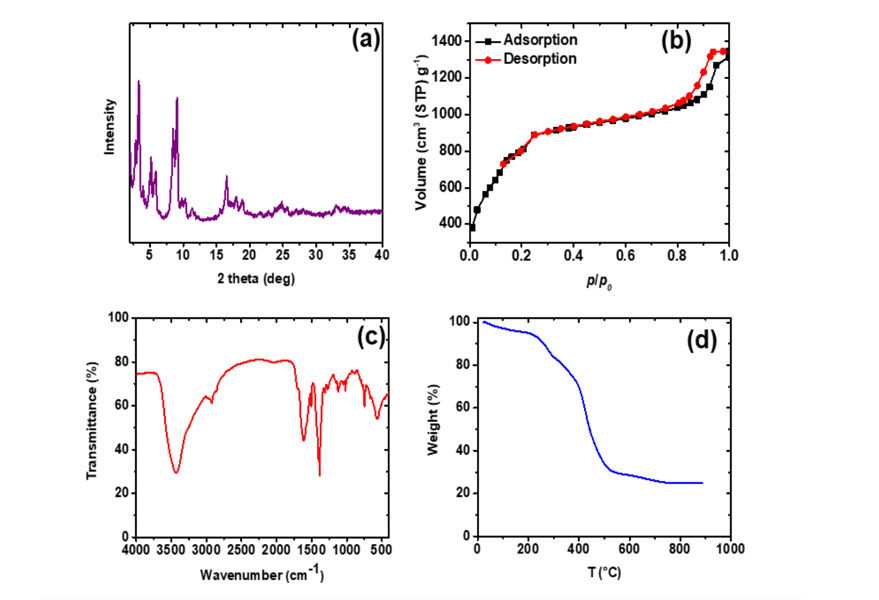
Fig. 2. XRD pattern (a), N2 adsorption-desorption isotherm at 77 K (b), FTIR spectroscopy analysis (c), and TGA thermogram under nitrogen atmosphere (d) of the synthesized MIL-101 (Cr).
2.Preparation of MIL-101 (Cr) based MMMs for gas separations
2.1PDMS&MIL-101 (Cr) MMMs
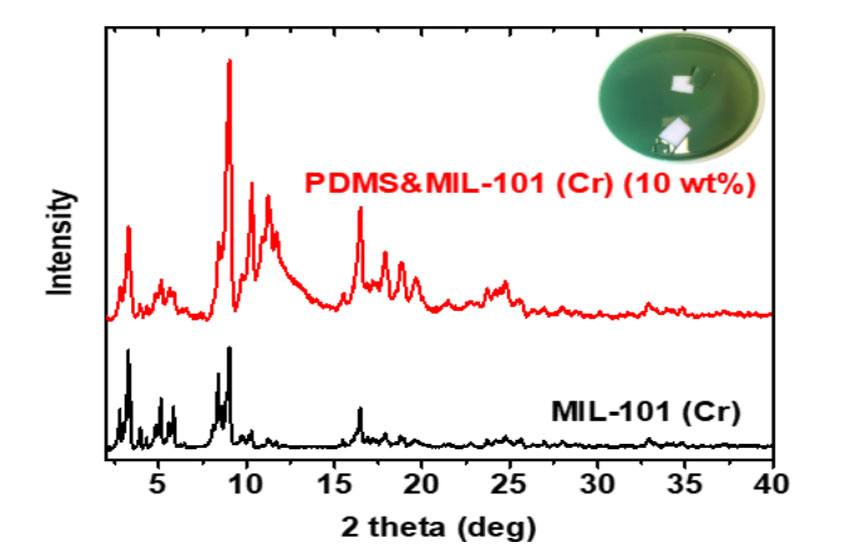
Fig. 3. XRD patterns of MIL-101 (Cr) nanoparticle and PDMS&MIL101 Cr) MMM with a particle loading of 10 wt%.
Poly(dimethylsiloxane) (PDMS) is used as the polymer for the MMMs because it currently has some of the best reverse-selective performance for a pure polymer [5]. The solvent evaporation technique was applied for preparing the pure PDMS and PDMS&MIL-101 (Cr) membranes. As shown in Fig. 3, the PDMS&MIL-101 (Cr) mixed matrix membrane shows a smooth surface and MIL-101 (Cr)-like green color. The XRD data of shows that the MIL-101 (Cr) pattern was relatively unchanged for the mixed matrix membrane (Fig. 3), suggesting that the MOF crystalline structure was preserved. In addition, the XRD pattern also indicates the MIL-101 (Cr) within PDMS polymers has no preferred orientation.
References
- Férey, G., et al., A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science, 2005. 309(5743): p. 2040-2042.
- Herm, Z.R., E.D. Bloch, and J.R. Long, Hydrocarbon separations in metal–organic frameworks. Chemistry of Materials, 2013. 26(1): p. 323-338.
- Alqaheem, Y., et al., Polymeric gas-separation membranes for petroleum refining. International Journal of Polymer Science, 2017. 2017.
- L. Bromberg, Y. Diao, H. Wu, S. A. Speakman, T. A. Hatton, Chromium(III) Terephthalate Metal Organic Framework (MIL-101): HF-Free Synthesis, Structure, Polyoxometalate Composites, and Catalytic Properties, Chem. Mater. 2012, 24, 1664−1675.
- Alqaheem, Y., et al., Polymeric gas-separation membranes for petroleum refining. International Journal of Polymer Science, 2017.
- G. Dong, H. Li, V. Chen, Challenges and opportunities for mixed-matrix membranes for gas separation, J. Mater. Chem. A 1 (2013) 4610–4630.
